Seamless onboarding, documentation arrived within the first shipment, and replenishment cadence locked in during week one. Strong visibility and support end-to-end.

- SKU
- LAC2228033
- Category
- Uncategorized
FAQs
What is the retail return or exchange policy on this item?
Unopened retail units are eligible for refund or exchange within 30 days of receipt. Initiate a return through your order history or call our retail support team, and we will email a prepaid return label within one business day.
How fast will my order arrive and which warehouse will ship it?
Orders release within 24 hours and ship from the LAC warehouse closest to your ZIP (Chicago, Dallas, or Los Angeles). Most retail customers receive delivery in 1–3 business days with full tracking updates.
Can I qualify for discounts or auto-refill pricing?
Yes. Add two or more cases, enroll in Subscribe & Save, or share your monthly usage and our retail team will apply bundle pricing instantly—no contracts required.
Which payment methods are supported for retail checkout?
We accept all major credit and debit cards, digital wallets (Apple Pay, Google Pay), Buy Now Pay Later, and HSA/FSA cards. ACH and wire options are also available for larger storefront purchases.
What kind of support do I get after purchasing?
Retail customer care is available 7 days a week by phone, chat, or email. Need product guidance, reorder help, or claim assistance? Text or call 844-LAC-6091 and we will respond within an hour.
Cook Medical G53704 - SET, STENT, URETERAL, SOFT, 5FX18CM, US-600-R, EACH
Product Description Cook Medical G53704 – SET, STENT, URETERAL, SOFT, 5FX18CM, US-600-R, EACH Universa Soft Ureteral Stent and Positioner Stent and positioner – (No wire guide) The Universa Soft Ureteral Stents and Stent Sets are flexible tubular devices made from radiopaque polyurethane.
Cook Medical G53704 – SET, STENT, URETERAL, SOFT, 5FX18CM, US-600-R, EACH

Universa Soft Ureteral Stent and Positioner
Stent and positioner – (No wire guide)
The Universa Soft Ureteral Stents and Stent Sets are flexible tubular devices made from radiopaque polyurethane. The stents are available with or without hydrophilic coating. Each end of the Universa Soft Ureteral Stent consists of a curled, tapered pigtail. Sideports are placed on each end of the stent, including on the pigtails of the stent. A braided or monofilament tether for repositioning and removal of the device is located on the proximal pigtail of the stent. Radiopaque graduation marks are located on the stent to increase fluoroscopic visualization during stent advancement and placement.
The Universa Soft Ureteral Stent Set includes
- Radiopaque double pigtail stent
- Stainless steel wire guide
- Radiopaque stent positioner with radiopaque tip
- Pigtail straightener
| Order Number | Reference Part Number | Fr | Length (cm) |
| G53704 | US-600-R | 6.0 | 22-32 |
Experience Universa
Universa stents provide temporary internal drainage from the ureteropelvic junction to the bladder. Universa products have premium features and the everyday advantage of a full product line. Consolidated part numbers enable streamlined ordering and provide cost savings. The Universa Soft stent offers a softer feel for patient comfort.
Features and Benefits

Comparison
Enlarged cross-section comparison of a typical 6 Fr stent and 5 Fr Universa stent
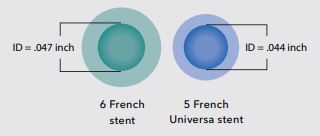
Intended Use
The Universa Soft Ureteral Stents and Stent Sets are used for temporary internal drainage from the ureteropelvic junction to the bladder. Ureteral stents have been used to relieve obstruction in a variety of benign, malignant and post-traumatic conditions. The stents may be placed using endoscopic, percutaneous or open surgical techniques.
Precautions
- The potential effects of phthalates on pregnant/nursing women or children have not been fully characterized and there may be concern for the reproductive and developmental effects.
- The Universa Soft Stents must not remain indwelling more than six months. If the patients status permits, the stent may be replaced with a new stent.
- These stents are not intended as permanent indwelling devices.
- The tether should be removed if the stent is to remain indwelling longer than 14 days.
- Do not force components during removal or replacement. Carefully remove the components if any resistance is encountered.
- A pregnant patient must be more closely monitored for possible stent encrustation due to calcium supplements.
- Improper handling can seriously weaken the stent. Acute bending or overstressing during placement may result in subsequent separation of the stent at the point of stress after a prolonged indwelling period.
- Angulation of the wire guide or stent should be avoided. Use of a 0-degree scope lens is recommended. Scopes larger than 21.0 French are suggested.
- Individual variations of interaction between stents and the urinary system are unpredictable.
- Ureteral stents should be checked periodically for signs of encrustation and proper function. Periodic checks of the stent by cystoscopic and / or radiographic procedures are recommended at intervals deemed to be appropriate by the physician in consideration of the individual patients condition and other patient specific factors. The stent is not intended as a permanent indwelling device which should not exceed 180 days.
- Use of the device should be based upon consideration of risk-benefit factors as they apply to your patient.
Potential Adverse Events
Complications of ureteral stent placement are documented. These complications include, but are not limited to:
- Extravasations
- Occlusion
- Migration
- Hemorrhage
- Sepsis
- Perforation of the urinary tract
- Peritonitis
- Encrustation
- Urinary tract infection
- Loss of renal function.
Product Recommendations
If no wire guide is provided with this set, the following is recommended:
- 5.0 French stents accept .035″
- 6.0 French stents accept .038″
- 7.0 French stents accept .038″
- 8.0 French stents accept .038″
MRI Information
Nonclinical testing has demonstrated that the Universa Ureteral Soft Stents are MR Conditional according to ASTM F2503. A patient with this device may be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 1.5 tesla or 3.0 tesla only
- Maximum spatial gradient magnetic field of 1600 gauss/cm (16.0 T/m) or less
- Maximum MR system reported, whole-body-averaged specific absorption rate (SAR) of
$13.46

Volume pricing, managed services, and consignment programs available through LAC Health procurement.
Contract & blanket PO support
GHX • Workday • SAP integrations
Delivery & Shipping
Check ETA for your dock in seconds.
Free standard shipping on orders over $75
Express and white-glove freight options available
Let’s architect a resilient supply programme together.
Invite our procurement strategists into your planning room and we’ll deliver a clinically-aligned sourcing roadmap—complete with compliance, telemetry, and logistics tuned to your realities.
Customer reviews
What procurement leaders and clinical teams are saying after deploying this SKU.
Arrived cold-chain validated with serialized lots pre-registered in our ERP. Teams aligned rapidly thanks to LAC’s transition kit and consultative launch.
