Product Description For 3M 9536HP
3M 9536HP – Dressing Tegaderm HP Oval 4×4-3/4″ Film Transparent Adh 50/Bx, 4 BX/CA

3M Tegaderm HP (Holding Power) Transparent Film Dressing Frame Style 9536HP, 4 in. x 4 in. (10 cm x 12 cm) 50/Box, 4Box/Case
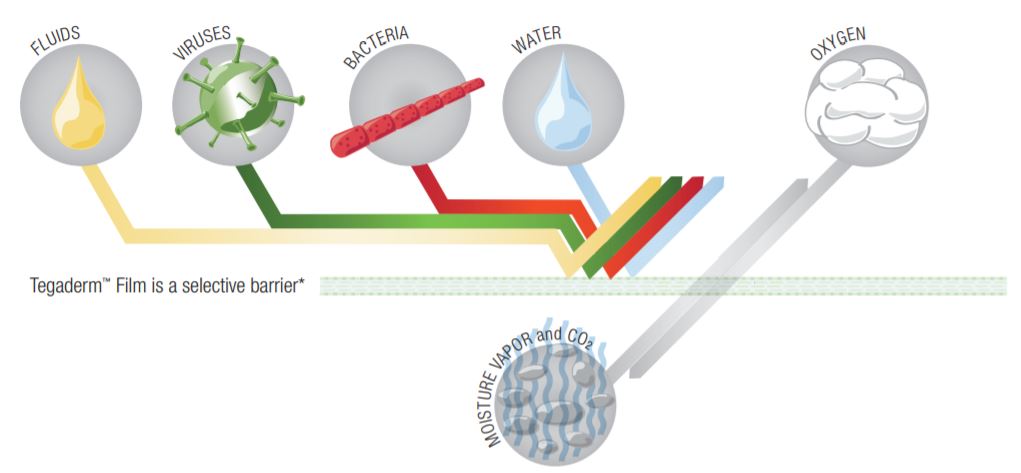
Tegaderm Film consists of a thin film backing with a non-latex, hypoallergenic adhesive. Tegaderm Film with Border is notched and reinforced with soft cloth tape to provide a better seal around catheters and other devices. The dressing is breathable, allowing good oxygen and moisture vapor exchange. It is waterproof and impermeable to liquids, bacteria, and viruses. An intact dressing protects the site from outside contamination.
- High adhesion keeps dressings in place under challenging conditions with 7 day wear time.
- Provides a waterproof, sterile barrier to external contaminants including liquids, bacteria and viruses. In vitro testing shows that the transparent film provides a viral barrier from viruses 27 nm in diameter or larger while the dressing remains intact w.
- Transparent dressing allows for continuous visibility to the I.V. insertion site.
- Picture-frame delivery makes accurate placement easy.
3M Tegaderm HP Transparent Film Dressings consist of a thin film backing with a hypoallergenic, latex-free adhesive that gently, yet securely, adheres to skin. Tegaderm dressings are breathable, sterile, transparent and waterproof, and provide a barrier to external contaminants. Tegaderm HP Film has a special adhesive for greater holding power in the presence of moisture. Specially designed Tegaderm dressings, with unique shapes and securing tapes provide solutions for difficult to dress wounds and I.V. catheters.
Tegaderm HP Transparent Film Dressing for improved adhesion in challenging conditions. Transparent dressing with extra holding power for challenging conditions including skin with moderate amounts of moisture.
Features and Benefits
Versatileone – Product to Satisfy Many Clinical Situations
Tegaderm dressings can be used to protect I.V. sites, enhance wound healing, prevent skin breakdown, and protect clean, closed surgical incisions. Tegaderm dressings are available in many sizes, shapes and application styles to meet a wide variety of needs. The frame allows the dressings to be tailored for special applications, when desired. Application systems of most other transparent dressings do not allow for this customization.
Easy to Apply – Unique Frame Delivery System
Application of Tegaderm dressing is intuitive and quick, making it easy to remember and easy to teach. It is especially convenient for patient self-care. Tegaderm dressing minimizes application time and saves dressing waste and costs. The frame delivery system provides maximum control of the thin film for rapid application of even the largest
dressings. The unique picture-frame allows precise and secure placement of the dressing every time. If the adhesive surface accidentally touches itself, the dressing can be separated and applied, eliminating wasted dressings. Tegaderm dressing is also available in a first-aid style delivery system for the health care professional who prefers this application method.
Gentle Adhesive – Just the Right Balance in Adhesive Strength
Tegaderm dressings are made with a hypoallergenic, latex-free adhesive that is gentle to the skin, yet securely holds catheters and other devices in place. Tegaderm dressing provides good initial adhesion without building to excessive levels over time. Even for dressings left in place for extended periods, the risk of patient discomfort and skin trauma is minimal when the dressing is properly removed. The hydrophilic nature of the Tegaderm HP Film adhesive makes it exceptionally adherent and useful for moist conditions and difficult-to-dress areas. It provides extra holding power, reducing unscheduled dressing changes.
Breathable – Lets Oxygen In and Moisture Vapor Out
The breathability of Tegaderm dressings allows moisture vapor and gas exchange, which is essential to maintain normal skin function under the dressing. Patients can wear Tegaderm dressings for extended periods of time, with minimal risk of skin irritation or maceration, and without excessive proliferation of skin flora
Waterproof, Sterile Barrier – Impervious To Liquids, Bacteria and Viruses
Tegaderm dressing acts as a barrier to protect the I.V. site or wound from external contaminants such as bacteria, viruses, blood and body fluids. Because Tegaderm dressings are waterproof, patients may bathe, shower or swim, if the dressing is completely sealed around the catheter or wound. Tegaderm dressing is sterile and remains so as long as the outer package is intact. Do not resterilize by gamma, steam, or E-beam.
Conformable – Flexes with Skin for Greater Patient Comfort
3M Tegaderm Transparent Film Dressing conforms to body contours, stretches easily, and prevents stress on the skin when the patient moves. It protects skin and bony prominences from abrasion, and allows the patient to move easily. Tegaderm dressing is comfortable to wear and presents a flat profile. The special shapes of Tegaderm dressings conform easily on difficult-to-dress areas, such as jugular and PICC insertion sites, and sacral wounds.
Enhanced Wound Healing – Improved Outcomes and Patient Comfort
Tegaderm dressing seals in natural wound fluid to maintain a moist environment, which has been shown to enhance the healing process. It prevents scab formation and dehydration of the wound bed, which can occur with conventional dry dressings. Tegaderm dressing provides a wound environment that allows epithelial cells to migrate easily across the wound surface, reducing pain caused by wound dehydration, and increases patient comfort.
Transparent – Allows Wounds and I.V. Sites to be Easily Monitored
The transparency of Tegaderm dressing provides complete visibility of the site during application. It also allows continuous monitoring of the I.V. site or wound without disturbing or removing the dressing. This visualization eliminates unnecessary dressing changes and saves nursing time.
Fewer Dressing Changes – Increased Patient Comfort
With Tegaderm dressing, fewer dressing changes mean improved patient comfort and the risk of skin trauma from repeated adhesive removal is reduced. For I.V. care, fewer dressing changes result in less catheter manipulation, and reduced exposure to potential outside contaminants. For wound applications, the longer wear time of Tegaderm dressing allows the wound to remain undisturbed, thereby preventing disruption of the healing process.
Affordable – Can be Worn Longer than Tape and Gauze Dressings
The HICPAC/CDC Guideline for the Prevention of Intravascular Catheter-Related Infection supports longer wear times for transparent dressings than tape and gauze dressings for I.V. applications.13 Less frequent dressing changes save nursing time and supply costs such as preps, gloves, and dressing materials.
Clinically Proven – Efficacious For I.V. Site and Wound Care
Numerous clinical trials by independent researchers support the use of Tegaderm transparent dressings for both I.V. site and wound care. Internationally recognized I.V. guidelines base site care recommendations on clinical studies using Tegaderm dressings. Most of the major I.V. studies have been conducted on high-risk patients with central catheters. The results of the largest, prospective randomized trials of central line dressings have proven that Tegaderm dressings are as safe as gauze and tape, even when worn for longer periods of time. The extended wear times of Tegaderm dressings did not increase the risk of I.V.
catheter-related bacteremias. Several studies of peripheral I.V. catheters have also been conducted. The largest of these, with 2088 catheters, demonstrated the safety and cost-effectiveness of Tegaderm dressings left in place for the duration of catheterization. Wound studies have demonstrated the importance of transparent dressings for rapid healing, protection of the wound from external contamination, and patient comfort. Clinical dossiers, one for the use of Tegaderm dressings for I.V. Therapy and the other for Wound Care, are available from your 3M representative upon request.
Radiologically Transparent
Tegaderm dressing is radiologically transparent. Removing the dressing from a patient prior to x-ray is not necessary.
Physical Properties/Definitions
Semi-Occlusive (Semi – Permeable)
Tegaderm and Tegaderm HP Transparent Film Dressings are made of semi-permeable films. They can be thought of as selective filters – they are occlusive to liquids, bacteria, and viruses; yet water vapor, oxygen, and carbon dioxide can easily be exchanged. Tegaderm and Tegaderm HP Film dressings are breathable. The breathability of a material is generally described in terms of oxygen and moisture vapor transmission rates (MVTR). Both rates are determined by the amount of gas that travels through the dressing in a given period of time, under specific conditions of temperature and humidity.
MVTR (Moisture Vapor Transmission Rate)
Moisture vapor transmission rate (MVTR) is the measurement of water vapor diffusion through a material. Two laboratory test methods are commonly used to measure MVTR. The results of these two tests are often used to compare transparent dressings for I.V. use. However, they do not represent real life conditions, and numerous variables can impact the results. This raises the question of whether laboratory test data for MVTR can accurately predict dressing performance in clinical practice. The inverted beaker test produces higher numbers with greater variability. These variances are seen
within samples of the same dressing, as well as among different products. This inconsistency occurs because the films can stretch and swell due to the water pressure against the test dressing, increasing the surface area measured. The MVTR values produced by the upright method are lower and more consistent among different products, and within samples of the same dressing. Because the liquid does not come in contact with the film in this test method, stretch and swell are not factors in the results. Aside from the test method chosen, many other variables can dramatically affect moisture vapor transmission rates.
- Volume of liquid in the test beaker (generally 1050 ml)
- Type of liquid medium (water, saline)
- Concentration of substances in the liquid (salt, proteins)
- Environmental conditions (temperature, humidity
MVTR bench tests are generally performed under tightly controlled temperatures and low relative humidity. In clinical settings, where temperatures and humidity vary considerably from typical test conditions, MVTR numbers will be much different than those produced in the laboratory. For example, under conditions of high humidity, moisture vapor transmission will proceed at a much slower rate. A third, less common method, uses computerized evaporimetry to measure moisture handling properties of transparent dressings. This instrument records actual evaporation through the film on skin, and moisture build-up underneath the dressing. When moisture vapor transmission is measured with this device, dressings with significant differences in bench MVTRs show no significant difference in actual moisture accumulation on the skin.17 Research studies have been conducted to investigate the effect of MVTR on clinical outcomes for I.V. therapy. The results of these trials do not demonstrate a correlation between higher MVTR and lower incidence of complications, including catheter-related bacteremia.
The Clear Choice for Improved Patient Care!
Tegaderm transparent dressings are preferred by clinicians for protecting and securing IV catheters and are safe for wound care applications. Product safety and performance is supported by clinical studies in a wide variety of clinical settings. 3M offers a broad line of sizes, shapes, and delivery systems, providing the clinician and patient with a solution for every transparent dressing need and are safe for wound care applications.
Precautions
- Hemostasis of the catheter site or wound should be achieved before applying the dressing.
- Do not stretch the dressing during application. Mechanical skin trauma may result if the dressing is applied with tension.
- Tegaderm transparent dressings should not be re-sterilized by gamma, E-beam or steam methods.
- Antimicrobial ointments containing polyethylene glycols may compromise the strength of Tegaderm HP Transparent Film Dressing.
Suggested Applications
- To cover and protect I.V. catheter sites
- Clean, closed surgical incisions
- Skin graft donor sites
- Skin tears and minor excisions
- Superficial partial thickness burns
- Stage I or II pressure ulcers
- Autolytic debridement facilitated by the moist wound healing environment
- Protective eyelid coverings
- Post tattoo application and removal.
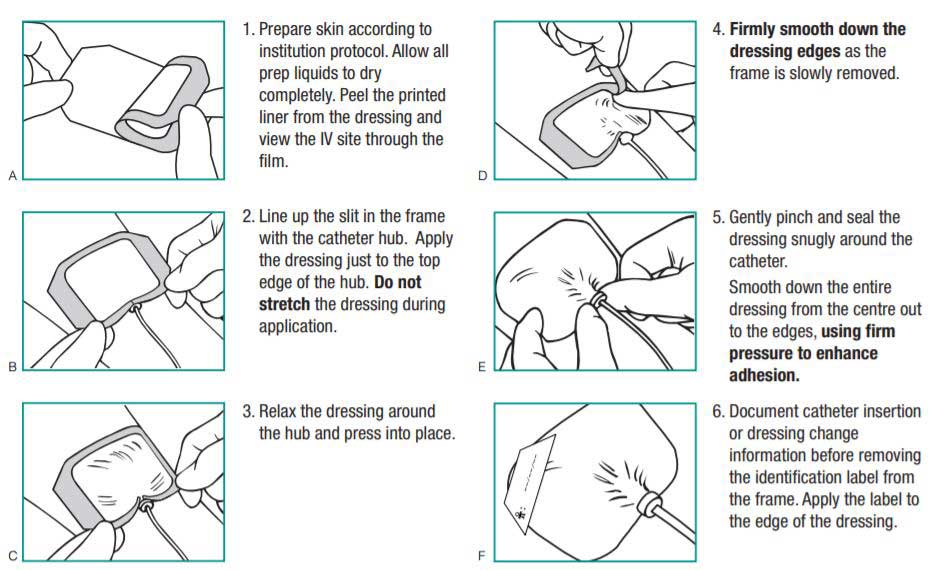
Application and Removal Instructions
Applying 3M Tegaderm Transparent Film Dressings
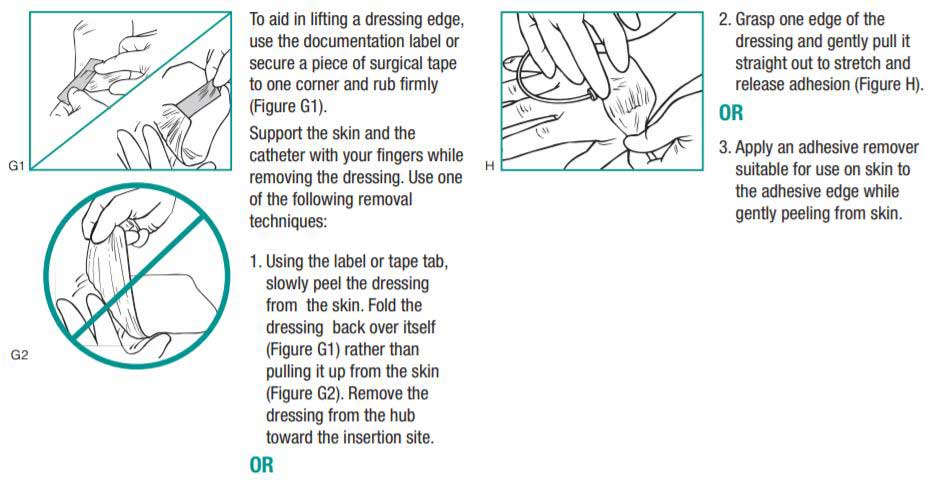
Removing 3M Tegaderm Transparent Film Dressings
Commonly Asked Questions
Question: What is MVTR? And, why does it matter?
Answer: MVTR (Moisture Vapor Transmission Rate) of a transparent film is measured in a Lab for purposes of materials research. The MVTR number does not predict the performance of a film dressing when on skin or in the clinical setting. This can be validated with an evaporimeter and with published clinical studies. (Clinical studies available upon request.)
Question: The clinical data is old. What new information has been published?
Answer: The 2002 HICPAC/CDC committee used the most recent clinical study using Tegaderm Film (Rasero, 2000) to base the recommendation for transparent dressing wear-time on central venous catheter sites. Since this was a welldesigned study, the CDC classified the recommendation as IA (for implementation and strongly supported by well designed experimental clinical or epidemiological studies). Well-designed clinical studies live forever and until new data is available, conclusions remain. Clinical portfolio is available from 3M. (70- 2009-6290-3)
Question: How does Tegaderm Film work with preps?
Answer: Tegaderm Film has been used with CHG and iodine-based preps for greater than twenty years. Testing indicates these skin preps have no effect on the adhesion of Tegaderm Film. Skin preps are not allowed to dry prior to the application of a transparent dressing, contact dermatitis (skin redness) may occur.
Question: What is the wear time on Tegaderm Film?
Answer: I.V. Sites: Based on the 2002 HICPAC/CDC recommendation and the clinical outcomes of studies using Tegaderm Film:
- Transparent dressings can be safely left on peripheral venous catheters for the duration of catheter insertion without increasing the risk for thrombophlebitis (MMWR, pp. 7, 17)
- Replace dressings used on short-term CVC sites every 2 days for gauze dressings and at least every 7 days for transparent dressings, except in those pediatric patients in which the risk for dislodging the catheter outweighs the benefit of changing the dressing (MMWR pp. 17). (Quick Summary CDC Guidelines: 70-2009-0582-9)
Wounds: Leave on as long as possible until the dressing becomes compromised (i.e. edge lift, rolled or soiled) to prevent disruption of the healing process. Dressing generally changed when fluid is excessive, dressing is loose or routinely at 7 days.
Question: What difference do the new CDC guidelines make?
Answer: The CDC guidelines provide evidenced-based recommendations proven to reduce catheter-related infections and the high costs associated with CRBSIs (Catheter-Related Blood Stream Infections). Major areas of emphasis in the 2002 Guidelines include: education and training, maximal barrier precautions for inserting central venous catheters, use of 2% CHG skin antiseptic, proper handhygiene using waterless products, defined dressing change intervals for peripheral and central venous catheter sites, discourage routine CVC replacement, and use of antimicrobial/antiseptic coated CVCs in certain circumstances.
Question: What is the best dressing to use for “difficult” situations?
Answer: When selecting an appropriate dressing, consider:
- location on patient: neck or IJ-#1655; sacrum #9543HP
- skin conditions: moist Tegaderm HP Film
- devices: large bulky or high profile catheters/introducers #1650 or 1655
- implanted ports: #1616 or 1650
- problems: edge lift #1614/1616
Question: Isnt Tegaderm Film the same as it has always been, for the past 20 years?
Answer: Since the launch of Tegaderm Film, 3M has introduced an alternate adhesive and film dressing (Tegaderm HP Film), a dressing specifically shaped for the sacrum, dressings with reinforced notches to reduce mechanical stress (drag) from heavy catheters, dressings with borders designed to reduce edge lift and sterile tape strips designed to enhance securement. Tegaderm Roll can be used as a secondary dressing or as a primary dressing over intact skin. Tegaderm Roll provides all the benefits of surgical tape, while providing a waterproof, bacterial, viral* barrier.
*Laboratory testing has proven Tegaderm and Tegaderm HP dressings provide a viral barrier (HIV-1 and HBV) while dressings remain intact without leakage.
Question: What is recommended if additional adhesion is needed? (e.g., tape/tincture of Benzoin)
Answer: Avoid using Benzoin since it is a skin irritant. If you use Benzoin, apply only around the dressings edge. Use a bordered dressing (1610, 1614, 1616, 1655, 1650). Select Tegaderm HP Film which has a hydrophilic adhesive for moist skin conditions.
Question: Any suggestions for patients with skin sensitivities?
- Allow preps to dry.
- Consider the use of a barrier film such as 3M Cavilon No Sting Barrier Film.
- Proper techniques for application: allow dressing to relax, pinch film onto device and apply pressure so that the dressing sticks to the skin. Apply pressure to dressing with your finger as you remove the frame and smooth down the dressing. The use of optimal removal technique (low and slow or stretch technique) will help to reduce discomfort. Maintain skin integrity and prevent the worsening of a current skin injury if it exists.
- Alternate adhesive (Tegaderm and Tegaderm HP Films have two different adhesive systems).
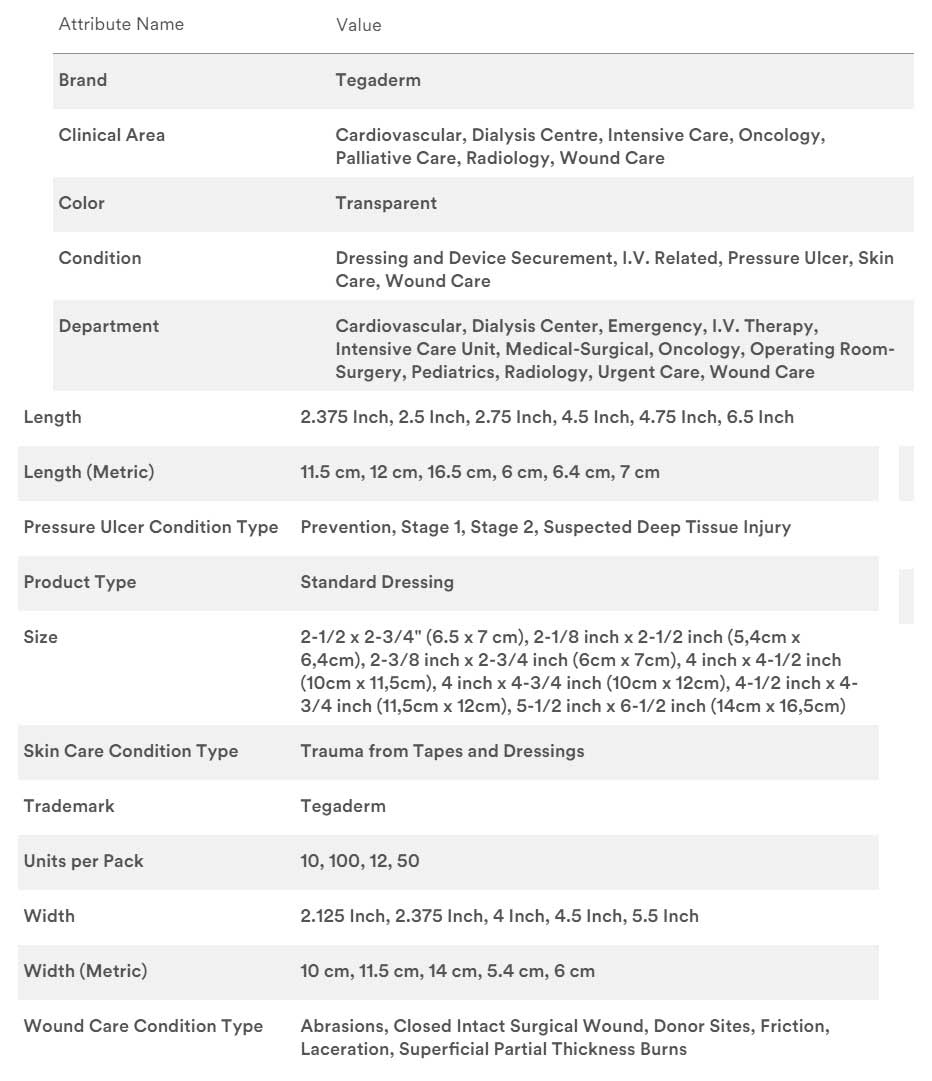
Specifications
CIA Medical always has the best pricing and availability for 3M 9536HP.












